BioSafe
Calcis Pond Lime 2.5 gal
- UPC:
- 850030517777
- Availability:
- Usually ships within 24 hours
- Shipping:
- Calculated at Checkout
Description
Calcis Liquid Pond Lime 2.5 gallons
Simplify liming a pond with Calcis, a liquid lime additive. Calcis is used to improve carbonate hardness in ponds.
5 gallons of Calcis is equivalent to 1 ton of quality agricultural lime

Liquid Lime Application Rate
Apply 2 gallons per acre-foot of water to increase Alkalinity/Carbonate Hardness by 10 mg/l
Calculate how much pond lime I need
What is the purpose of Calcis?
Calcis increases water hardness and alkalinity. Adequate hardness and alkalinity levels reduce harmful pH fluctuations.
When should Calcis be used?
Use Calcis in fishing ponds with low alkalinity. *
*Water Testing is recommended prior to pond lime treatment. Treatment rates are based on current alkalinity and desired alkalinity.
What is Alkalinity?
Alkalinity is an important measure of the capacity of a body of water to neutralize or buffer acids, and is expressed in terms of milligrams per liter or parts per million calcium carbonate (mg/L or ppm CaCO3). It is essential for good pond productivity, with a desirable range for fish culture between 75 and 200 mg/L CaCO3. Carbonates and bicarbonates are generally the major contributors to alkalinity, which is produced primarily when carbon dioxide (CO2) interacts with water and limestone. Rainwater absorbs atmospheric CO2 as it falls to the earth, becoming naturally acidic and lowering its pH. Similarly, well water has high levels of CO2 due to bacterial processes in soils and underground mineral formations, resulting in low pH and oxygen concentrations.
As ground- or rainwater moves over and through soil containing calcitic limestone (CaCO3)or dolomitic limestone [CaMg(CO3)2], the acidity caused by CO2 will dissolve the limestone, forming calcium and magnesium bicarbonate salts that raise alkalinity levels as well as pH and hardness. The products of this reaction are calcium hydrogencarbonate (CaHCO3), magnesium hydrogencarbonate (MgHCO3), calcium bicarbonate (Ca(HCO3)2), magnesium bicarbonate (Mg(HCO3)2), calcium carbonate (CaCO3) or magnesium carbonate (MgCO3). These chemicals act like sponges, absorbing hydrogen ions from acids before they can lower the pH too drastically, creating a more stable environment for aquatic life.
What does lime do for a pond?
Adding lime to a pond improves conditions for fish and other aquatic life. Liming a pond improves fish growth and reproduction. Lime improves water quality which improves overall fish health.
Another lessor known reason for liming is improved pond treatment effectiveness. Ponds with low pH and alkalinity may see limited results from some herbicides/algaecides as well as beneficial bacteria treatments.
How to lime a pond
- Know your pond's alkalinity and estimated water volume before application
- Use the Calculator below to determine how much lime is needed - On average, 1 ton or more of agricultural lime is required per acre (5 gallons of Calcis is equivalent to 1 ton of QUALITY agricultural lime)
- Pour Calcis throughout pond or in areas with greatest amount of flow
- Retest alkalinity 1 week after application to ensure levels are adequate
- Frequency of application is dependent on run off, inputs, rainfall, replenishment from water well, etc.
Calcis vs Agricultural Limestone
Calcis is produced from the highest quality lime. Other bulk lime may or may not be of equal quality. Calcis is in liquid form making it easy to apply and produces faster results.
Agricultural lime comes in powder form. To ensure distribution, powdered lime needs to be distributed throughout the entire pond.
Why is Pond Lime Important to Water Quality?
1. To Raise and buffer the pH
One of the primary reasons why pond liming is important is that it can help to raise and stabilize the pH of the water. The pH is a measure of how acidic or basic a substance is, and water with a higher pH is less likely to be affected by pollutants.
The quality of water in fish ponds is an important factor for the health and productivity of the pond inhabitants. In order to maintain optimal conditions, it is essential to monitor a range of chemical components that are interrelated and can have a significant impact on various aspects of the environment. Alkalinity, pH, and hardness are all key parameters to consider when assessing the water quality of a pond. These values should be monitored regularly as they can fluctuate or cycle daily and also change over time depending upon the mineral content of the surrounding environment. A regular analysis of these chemical components will ensure that fish ponds are maintained at ideal levels for optimal health, growth and productivity.
In addition to water quality, the oxygen availability of a pond is also important for fish health and productivity. Dissolved oxygen levels can be affected by several factors such as temperature, pH, alkalinity and turbidity. High temperatures reduce the amount of dissolved oxygen available in the water while lower temperatures increase it. The pH level will have an impact on both the solubility of oxygen and the toxicity of ammonia. Alkalinity can help buffer against swings in pH while turbidity affects how much light is able to penetrate the water, which then impacts photosynthesis by aquatic plants and thus dissolved oxygen availability.
2. To Improve Fish Health and Growth
Pond liming can also increase fish growth rates. This is because limestone raises the pH of the water, which makes it easier for fish to absorb nutrients from their food. Additionally, a higher pH can also help to reduce stress levels in fish, which can further improve growth rates.
Fish thrive in an optimal pH range between 6.5 and 8.5, and any alkaline values above 9.2 or acidic values below 4.8 can be damaging and even deadly to salmonids such as brown trout or rainbow trout. A few hardy species that can withstand acidity levels as low as 4.5-5.0, but the healthiest conditions for most fish species are near neutral to slightly basic.
Low pH levels are often prevalent during the spring months, especially when snow melts and releases acidified water, or in areas where peat bogs have drained into streams or ponds. High alkaline values can occur in eutrophic (nutrient-rich) reservoirs because of the high rate of photosynthesis by green plants such as blue-green algae, higher aquatic plants, etc., which take up significant amounts of carbon dioxide from surrounding waters with medium alkalinity levels.
The pH balance of a body of water can also be affected by mineral acids or hydroxides leeching from other sources, or from acidic or alkaline substances being discharged into rivers and lakes. Fish possess a natural defense against extreme pH levels, which involves them producing increased amounts of mucus that can be seen externally on their skin and gill covers. However, if the pH level is too high or low, it can cause damage to their tissues, particularly the gills.
In addition to creating an optimal environment for fish to live in, pH is also important in determining the toxicity of other substances such as ammonia, hydrogen sulphide, cyanides and heavy metals on fish. Therefore, monitoring and maintaining a balanced (buffered) pH level is essential for preventing serious health implications for fish living in artificial bodies of water.
In ponds and lakes with heavy plant and algae populations, CO2 generated by respiration at night can drive severe pH fluctuations over a 24 hour period. The fluctuating pH creates other water quality changes and can result in significant stress for aquatic life. When high rates of photosynthesis are occurring, it is especially important to buffer the water using lime or Calcis to prevent rapid pH swings.
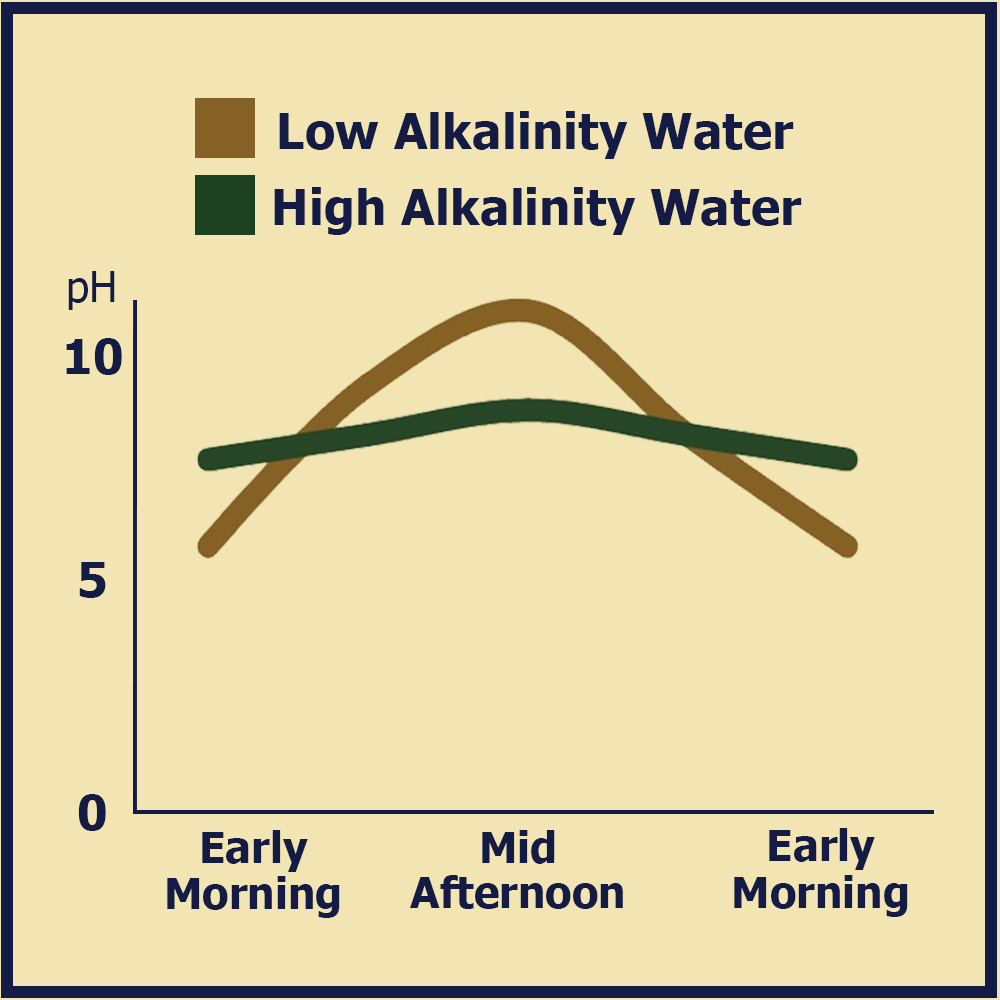
Alkalinity and Pond Productivity
By raising alkalinity levels above 20 milligrams per liter (mg/L), CO2 concentration is trapped and made available for photosynthesis, which increases the productivity of phytoplankton in a given body of water. As such, maintaining an alkalinity level above 20 mg/L supports optimal phytoplankton growth and helps sustain good pond fertility overall.
Phytoplankton are tiny single-cell aquatic plants, most of which are microscopic or near-microscopic in size. These organisms play a critical role in the environment of ponds by contributing to photosynthesis and primary productivity, as well as maintaining oxygen levels. In addition, their presence can also have an effect on pH balance and nutrient availability - two factors that contribute to pond fertility. In short, phytoplankton are integral to proper functioning ponds; they not only provide oxygen through photosynthesis but also enhance nutrient availability and improve alkalinity conditions that support productive ecosystems.
Calcium Hardness and Fish Health
Calcium and magnesium are indispensable elements in the biological processes of fish, such as the formation of bones and scales, blood clotting, and other metabolic reactions. Fish can get calcium and magnesium from their food or from the water itself. Of all the divalent salts present in fish culture water, calcium is one of the most critical components; this is because its presence helps to minimize the loss of other essential minerals like sodium and potassium through osmotic leakage from fish body fluids (e.g., blood). Sodium and potassium are both vital for heart, nerve and muscle function in fish. Experiments have revealed that adequate levels of free calcium in culture water also facilitate the re-absorption of lost salts by fish. Different species may require various concentrations of calcium hardness; for instance, red drum and striped bass will show better survival rate with a higher concentration (i.e., between 40 – 100 mg/L). In addition, rainbow trout can withstand low calcium levels if pH levels are above 6.5 (10 mg/L being a minimum level). To guarantee optimal health conditions for aquatic life, it is advisable to match the calcium concentration in their surrounding environment with that found in their blood (100 mg/L as CaCO3 hardness). We recommend conducting tests for calcium hardness on samples of potential fish culture waters before introducing any species into them. Apart from promoting healthy development through proper mineral balance inside the bodies of certain fish species, having an optimal level of environmental calcium also helps to conserve energy otherwise used to re-absorb lost salts. Consequently, making sure that fish are living in the right environment with adequate levels of calcium is very important in order to ensure good growth rates and improved survival.
3. To Improve Water Clarity
Pond liming can also help to improve water clarity by reducing the amount of suspended particles in the water. Suspended particles are small pieces of matter that float in the water and make it appear cloudy. These particles can come from a variety of sources, including algae, dirt, and debris. By raising the pH level of the water, pond liming can help to coagulate these particles so that they settle out of the water and improve its clarity.
4. To Enhance Plant Growth
Pond liming can also help to enhance plant growth. Aquatic plants require certain nutrients in order to grow properly, and these nutrients are often more available in water with a higher pH level. Additionally, the calcium present in limestone can help to improve plant health and encourage strong root growth.
*We typically respond to detailed calculation requests within 1 business day.





















